Background: Modern treatment goal for patients with chronic myeloid leukemia (CML) is to maintain treatment-free remission (TFR) following tyrosine kinase inhibitor (TKI) discontinuation. The control of residual leukemia cells may occur with immunological mechanisms as several studies have shown that a higher proportion of CD56 dim NK cells before TKI discontinuation is associated with a better probability of TFR. In addition, CD8+ T cell abundances and phenotypes have been associated with responses to TKIs. However, the more detailed phenotypes and mechanisms and how to translate these findings to increased TFR rates have remained unknown.
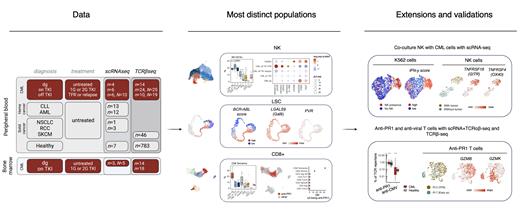
Methods: We analyzed 55 single-cell RNA and T cell receptor (TCR) sequenced (scRNA+TCRαβ-seq) samples from patients with CML ( n=13, N=25), other cancers (AML n=11, CLL n=13, solid tumors n=4), and healthy ( n=7). Additionally, we profiled 90 CML and 786 healthy TCRβ-seq samples, and sorted T cells specific to leukemia-associated antigen PR1 and matched these back to the scRNA+TCRαβ-seq and TCRβ-seq data. To validate and refine our findings, we performed co-culture of primary and expanded NK cells with CML cell lines (K562 and LAMA84) with a multiplexed scRNA-seq readout.
Results: In the pan-cancer scRNA+TCRαβ-seq comparison of >250,000 cells, CML was separated from other cancers and healthy by the high number and active phenotype NK cells. The other phenotype more pronounced in CML was CD8+ T EM/T EMRA phenotype.
Most NK cells in CML had an active CD56 dim phenotype with high expression of GZMA/B, PRF1, CCL3/4 and IFNG. In the off-TKI samples from patients with TFR, these NK cells upregulated NK cell activation genes such as TNFRSF4/OX40, TNFRSF9/4-1BB, and TNFRSF18/GITR. The most important predicted interactions between NK cells and CD34+ leukemic cells were inhibitory LGALS9- TIM3 and PVR- TIGIT interactions. Following TKI discontinuation, the inhibitory markers HAVCR2/TIM3 and TIGIT decreased in patients with TFR, which was not seen in patients with relapse following TFR.
Accordingly, in our co-culture data of NK cells with CML cell lines, we noted activation of NK cells to a similar phenotype as noted in ex vivo off-TKI samples. In co-culture with CML target cells, NK cells gained an activation by upregulation of genes like TNFRSF4/OX40, TNFRSF9/4-1BB, TNFRSF18/GITR and the inhibitory markers HAVCR2/TIM3 and TIGIT. In co-culture with NK cells, CML target cells upregulated the IFN-γ response pathway including elevated HLA-expression and accordingly, upregulation of LGALS9 and PVR.
The CD8+ T EM/EMRA cells that were expanded in CML diagnosis were suppressed following TKI treatment and decreased following TKI discontinuation. To track anti-CML T cells, we created a classifier to identify TCRs targeting leukemia-associated antigen PR1 from the TCRb-sequenced samples. As a validation of our classifier, we noticed that anti-PR1 T cells were more prevalent in CML than in healthy ( P adj<0.0001) or melanoma ( P adj<0.0001), more expanded than similar anti-viral T cells ( P adj<0.0001) and enriched in bone marrow samples compared to peripheral blood ( P adj<0.0001). From different TKIs, dasatinib expanded anti-PR1 T cells the most ( P adj<0.01).
In the scRNA+TCRαβ-seq data, anti-PR1 T cells were enriched in the mature, cytotoxic CD8+ T EMRA phenotype ( P adj<0.0001). In a patient maintaining TFR, anti-PR1 T cells had a highly cytotoxic profile ( GZMB, PRF1) throughout the therapy with upregulated NK receptors like FCGR3A/CD16 and KIR2DL2, even more so than T cells targeting other targets e.g., different viruses. In comparison, the anti-PR1 T cells in a patient with early relapse had an exhausted profile with upregulation of memory marker GZMK and exhaustion markers like LAG3 and HAVCR2/TIM3.
Conclusions: With our in-depth analysis of cellular and molecular immune responses in CML, we identified the active NK cells and anti-PR1 T cells that could help maintain TFR in patients discontinuing TKI treatment. Different immune-checkpoint blockade therapies such as anti-TIM3 could be considered as future strategies to improve the TFR rates.
Disclosures
Forstén:Orion Corportaion: Ended employment in the past 24 months. Dhapola:Nygen Analytics AB: Current Employment. Ilander:Brisol Myers Squibb: Current Employment. Olsson-Strömberg:Incyte: Honoraria. Hjorth-Hansen:Bristol Myers Squibb: Honoraria; Novartis: Honoraria; Incyte: Honoraria. Burchert:MSD: Research Funding; Incyte: Honoraria; Novartis: Honoraria, Research Funding. Karlsson:Nygen Analytics AB: Current Employment. Kreutzman:Bristol Myers Squibb: Current Employment. Mustjoki:Novartis: Honoraria, Research Funding; Pfizer: Research Funding; BMS: Honoraria, Research Funding; Dren Bio: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal